Discoveries
2. Making Better Catalysts
3. Understanding Chemical Reactions
4. New Types of Superconductors
5. Development of Neutron Scattering Facilities
6. Development of Synchrotron Radiation Light Sources
7. Development of Lithium Batteries
8. A New Class of Carbon Structures
9. Engineering Organisms to Make Valuable Biomaterials
10. Heavy Element Chemistry
11. Improving Intermetallic Compounds
12. Ion Beam Techniques Enhance Materials Science
13. Preventing Radioactive Contamination
14. Explaining and Applying Magnetism
16. Organic-Based Magnets: A New Frontier
17. Manipulating Light in Photonic Crystals
18. Extending the Power of Nuclear Magnetic Resonance Techniques
19. Saving the Earth's Ozone Layer
20. Making Solar Energy More Affordable
21. Enhancing Separations and Analysis
22. Sequencing the First Plant Genome
23. New Opto-electronic Materials and Devices
24. Unraveling the Mystery of High-Temperature Superconductivity
25. Harnessing the "Thermoacoustic" Effect
26. Metallic Glasses with Extraordinary Properties
27. Extending the Science of Transition Metal Nitrides
28. A New Type of Microscopy
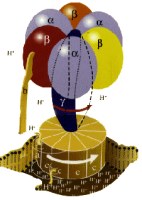
Enzymatic mechanism
of ATP synthesis
The energy cycle of all living organisms involves the molecule adenosine triphosphate (ATP), which captures the chemical energy released by the metabolism of nutrients and makes it available for cellular functions such as muscle contraction and transmission of nerve messages. A hard-working human adult can convert almost a ton of ATP daily. From the early 1960s through 1994, the Office of Science supported Paul D. Boyer's research at the University of California at Los Angeles on ATP synthase, the enzyme responsible for synthesizing ATP. His research examined the detailed chemical reactions involved in ATP synthesis and how the enzyme uses energy to create new ATP. Boyer theorized that this "molecular machine" with rotating parts functions in a surprising way for enzymes, a mechanism later supported by the work of John E. Walker of the United Kingdom. Among other things, Boyer discovered that energy input was not used primarily to form the ATP molecule, but rather to promote the release of an already formed and tightly bound ATP. Boyer and Walker shared half of the 1997 Nobel Prize in Chemistry for these achievements.
Scientific Impact: This work uncovered new concepts in enzymology and advanced understanding of how living cells function at the molecular level. Determination of how cells store and transfer energy has been among the most important advances in molecular and cell biology, enabling an entire generation of work at the cellular level in animal and plant research.
Social Impact: Research in cell biology has led to tremendous advances in medicine and physiology, such as clues to the genesis and treatment of cancer.
Reference: Boyer, P.D., "The ATP synthase—a splendid molecular machine," Ann Rev Biochem 997;66:717-49
Catalysts, which accelerate chemical reactions, are valuable in many industries, from fuels to pharmaceuticals. Long-term research by government and academic scientists supported by the Office of Science has led to new understanding of catalytic phenomena, in particular the relationship between chemical structure and reactivity. For example, early work established two classes of heterogenous catalysts (which function by adsorbing molecules), based on whether chemical reactivity is, or is not, sensitive to surface structure. These studies showed that catalytic reactions once thought to be structurally insensitive actually took place on a dynamic surface. Research on the reactivity of hydrogen with catalysts—an issue in the world's largest-scale industrial processes, such as sulfur removal from crude oil-disproved the widely held belief that hydrogen molecules must dissociate into two atoms before undergoing reactions, and challenged the accepted notion that surface-bound (as opposed to embedded) hydrogen was the only reactive form. Other discoveries concerned the chemical behavior of organometallic complexes (combinations of organic and metallic species) that are used, for example, in plastics manufacturing.
Scientific Impact: Research on structure-reactivity relationships has increased understanding of both natural and synthetic processes. The discovery of nonclassical binding of molecular hydrogen created a new field of study that may overcome some of chemistry's greatest challenges, such as conversion of natural gas to more usable liquid fuels (methanol or gasoline).
Social Impact: A modern society's standard of living can be measured by its accomplishments in catalysis, because every manufacturing process and energy-generating technique starts with catalysis. Catalysts first introduced by investigators supported by the Office of Science revolutionized a process used to make about 100 billion pounds of plastics per year worldwide; this work is leading to catalysts that produce superior plastics with new properties.
Reference: C.E. Tripa and J.T. Yates, Jr. Nature, 398 (1999) 591.
The molecular features that influence the rate of chemical reactions were poorly understood until the mid-1960s, when Dudley Herschbach and his postdoctoral student Yuan T. Lee began a series of experiments at Harvard University. With funding from the Office of Science and predecessor agencies, they explained in detail how chemical reactions take place, and solved the problem of how to observe the random directions and velocities of molecules in a gas or a liquid. They developed an apparatus in which crossed molecular beams were used to vary the velocities and approach angles of reacting molecules. With this tool, the scientists discovered and studied long-lived complexes of reactants formed before a reaction was completed, and described theoretically their formation and decay. They also examined the velocities of products, rotational energies, and internal vibration energies. In this way, they could map out all the details of a chemical reaction and explain the effects of temperature and pressure. Initially, these studies focused on reactions between alkali atoms and other molecules; Lee later adapted the crossed molecular beam method for general reactions. The 1986 Nobel Prize in Chemistry was awarded jointly to Herschbach, Lee, and a third scientist.
Scientific Impact: This work contributed significantly to the modern knowledge base for atmospheric and combustion chemistry. These scientists helped establish reaction dynamics as a discrete field of research, and their crossed molecular beam approach is among the most important contributions to this field.
Social Impact: These studies contributed to improvements in industrial production efficiency and assisted in the design of new products to be more useful, durable, and conserving of natural resources. This work also contributed to the development of predictive theories and models used to design and manufacture new products ranging from plastics to pharmaceuticals.
Reference: http://nobelprize.org/nobel_prizes/chemistry/laureates/1986/index.html
Superconductors conduct electricity with little or no resistance. Organic superconductors contain carbon and are less dense than their ceramic or metallic counterparts; they also offer unusual potential for fine-tuning of electrical properties. Argonne National Laboratory long has carried out the major U.S. effort to synthesize and identify organic superconductors. Nearly 100 new superconductors of this type have been produced, with critical temperatures (at which a superconductor loses all electrical resistance) as high as -260 degrees C, or -434 degrees F. Recently, the first superconductor composed entirely of organic components (with no metal atoms) was synthesized, with a transition temperature in this range. Although this remains far lower than the highest known transition temperature for ceramics, scientists still expect that a high-temperature organic superconductor may be possible, such that liquid nitrogen (at -196 degrees C, or -321 degrees F) could be used as the coolant instead of the more costly liquid helium, thus making practical applications more feasible. The new compound has a two-dimensional, layered structure, which may provide significant insight into the nature of superconductivity.
Scientific Impact: These advances will help scientists develop a theory of how organic superconductors work and contribute to the design of new materials with higher transition temperatures. The all-organic material is ideal for studies of magnetic and charge transport properties because there is no possibility of contamination from metallic impurities.
Social Impact: Superconductivity already has important applications, such as medical diagnostic equipment, and many more uses are possible if transition temperatures are high enough. The availability of purely organic superconductors greatly expands the possibilities, especially for applications in which weight is a factor.
Reference: Ambient-Pressure Superconductivity at 2.7 K and Higher Temperatures in Derivatives of beta(ET)2IBr2: Synthesis, Structure, and Detection of Superconductivity. Williams, J. M.; Wang, H. H.; Beno, M. A.; Emge, T. J.; Sowa, L. M.; Copps, P. T.; Behroozi, F.; Hall, L. N.; Carlson, K. D.; Crabtree, G. W. Inorg. Chem. 1984, 23, 3839-3841.
A New Ambient-Pressure Organic Superconductor, kappa (ET)2Cu[N(CN)2Br, with the Highest Transition Temperature Yet Observed ( Inductive Onset Tc=11.6 K, Resistive Onset=12.5 K) Kini, A. M.; Geiser, U.; Wang, H. H.; Carlson, K. D.; Williams, J. M.; Kwok, W. K.; Vandervoort, K. G.; Thompson, J. E.; Stupka, D. L.; Jung, D.; Whangbo, M.-H. Inorg. Chem. 1990, 29, 2555-2557.
From Semiconductor-Semiconductor Transition (42 K) to the Highest-Tc Organic Superconductor, kappa (ET)2Cu[N(CN)2Cl (Tc=12.5 K) Williams, J. M.; Kini, A. M.; Wang, H. H.; Carlson, K. D.; Geiser, U.; Montgomery, L. K.; Pyrka, G. J.; Watkins, D. M.; Kommers, J. M.; Boryschuk, S. J.; Strieby Crouch, A. V.; Kwok, W. K.; Schirber, J. E.; Overmyer, D. L.; Jung, D.; Whangbo, M.-H. Inorg. Chem. 1990, 29, 3272-3274.
The First Organic Cation-radical Salt Superconductor (Tc=4 K) with an Organometallic Anion: Superconductivity, Synthesis and Structure of kappa (ET)2M(CF3)4(C2H3X3). Schlueter, J. A.; Geiser, U.; Williams, J. M.; Wang, H. H.; Kwok, W. K.;Fendrich, J. A.; Carlson, K. D.; Achenbach, C. A.; Dudek, J. D.; Naumann, D.; Roy, T.; Schirber, J. E.; Bayless, W. R. J. Chem. Soc., Chem. Commun. 1994, 1599-1600.
Superconductivity at 5.2 K in an Electron Donor Radical Salt of Bis (ethylenedithio) tetrathiafulvalene (BEDT-TTF) with the Novel Polyfluorinated Organic Anion beta (ET)2SF5CH2CF2SO3) Geiser, U.; Schlueter, J. A.; Wang, H. H.; Kini, A. M.; Williams, J. M.; Sche, P. P.; Zakowicz, H. I.; VanZile, M. L.; Dudek, J. D.; Nixon, P. G.; Winter, R.W.; Gard, G. L.; Ren, J.; Whangbo, M.-H. J. Am. Chem. Soc. 1996, 118, 9996-9997.

Neutron scattering provides key information on the positions, motions, and magnetic properties of solids. When neutrons flowing from a nuclear reactor bounce off atoms in a sample, the neutrons scatter in directions that depend on the atoms' relative positions in the sample structure. Changes in the neutrons' velocity provide information on the atoms' oscillations, or dynamics. Since the late 1940s, the Office of Science and predecessors have been major supporters of neuron science, including work by Clifford Shull and Bertram Brockhouse, who shared the 1994 Nobel Prize in Physics for their development of neutron scattering techniques for studies of condensed matter. Researchers at Oak Ridge, Brookhaven, and Argonne national laboratories developed neutron sources for spectroscopy, scattering, and imaging experiments and helped pioneer most of the associated instruments and techniques. The Office of Science currently supports three neutron sources—the High-Flux Isotope Reactor at Oak Ridge National Laboratory, Intense Pulsed Neutron Source at Argonne National Laboratory, and Manuel Lujan Jr. Neutron Scattering Facility at Los Alamos National Laboratory—used by hundreds of researchers annually. Under construction is a spallation neutron source at Oak Ridge that will be about an order of magnitude more powerful than any existing pulsed neutron source. Spallation produces neutrons with little heat; pulsed operation provides very high peak intensities.
Scientific Impact: Neutrons' unique properties, such as sensitivity to light elements, make them invaluable tools for polymer, biological, and pharmaceutical sciences. Studies made possible by neutron sources and the associated techniques contribute to the development of new materials, such as ceramic superconductors.
Social Impact: Neutron studies lead to new and improved products, such as powerful magnets for highly efficient electric motors. Also, because their high penetrating power allows nondestructive property measurements deep within a specimen, neutrons have been used to examine automotive gears and brake discs, and defects in aircraft wings, engines, and turbine blades.
Reference: Scientific Research Facilities: A National Resource, Office of Basic Energy Sciences, /bes/recent-bes-information-brochures
Synchrotrons produce a unique type of radiation—continuous across the spectrum and tunable to the desired wavelength—emitted by electrons accelerated in a magnetic field. For two decades, the Office of Science has been the major supporter of U.S. synchrotron light sources. It currently operates four, each with unique capabilities, used by a total of more than 6,000 researchers annually from academia, government, and industry. The four are the Advanced Light Source at Lawrence Berkeley National Laboratory, Advanced Photon Source at Argonne National Laboratory, National Synchrotron Light Source at Brookhaven National Laboratory, and Stanford Synchrotron Radiation Laboratory at Stanford Linear Accelerator Center. Scientists at these sites helped pioneer many synchrotron innovations that are widely used today, including a lattice of magnets that increased brightness (photon density) by two orders of magnitude; "insertion" devices (linear arrays of magnets called wigglers and undulators) that oscillate the path of the electron beam to generate X-ray and ultraviolet light that is high in flux (number of photons) and collimation (parallel alignment of photons); and powerful experimental techniques such as X-ray scattering and X-ray microscopy.
Scientific Impact: These innovations made new science possible and paved the way for significant extensions of light source performance that have had a broad and deep impact on the understanding of matter. Synchrotrons are used for cutting-edge research in materials science, physical and chemical science, geosciences, environmental science, bioscience, and medical and pharmaceutical science.
Social Impact: Synchrotron research affects society in areas such as information and energy technologies. For example, recent high-resolution imaging of thin films of copper may assist in the development of ultrahigh-density computer hard drives, and imaging of contaminants in solar cells and their removal by heat treatment may lead to more efficient and less costly solar energy.
Reference: Scientific Research Facilities: A National Resource, Office of Basic Energy Sciences, /bes/recent-bes-information-brochures
P. A. Montano and H. Oyanagi, "In Situ Synchrotron Radiation research in Materials Science," MRS Bulletin, (24) 13-20 (January 1999).
W.Yun et al., "S-ray Imaging and Microspectroscopy of Plants and Fungi," J. Synchrotron Rad., (5) 1390-1395 (1998).
Lithium batteries, which offer both high energy-storage capacity and an environmentally benign alternative to the harmful lead used in conventional batteries, are based on research supported by the Office of Science and its predecessors. An early innovation was the development of organic solid electrolytes—essential because traditional water-based electrolytes could react with metals such as lithium to cause an explosion. (A battery consists of positive and negative electrodes separated by an electrolyte, through which ions, or charged atoms, flow.) Charles Tobias at Lawrence Berkeley National Laboratory led the search for nonaqueous solutions from which reactive metals, such as lithium (then used in fusion-type nuclear weapons), could be electrolytically deposited. He focused on cylic esters, including propylene carbonate, which today is used extensively in battery technology. The pioneering research included the purification of solvents to dissolve the electrolyte, solubility and conductivity measurements, and decomposition and electrodeposition tests. The Office of Science currently supports research on ion transport in solid polymer and glassy electrolyte systems, helping to lay the groundwork for the next generation of highly efficient and environmentally friendly batteries and fuel cells.
Scientific Impact: Tobias is widely regarded as the father of electrochemical engineering because he introduced scientific methods into a field formerly characterized by trial and error. His initial characterization of nonaqueous electrolytes, and demonstration that reactive metals could be electrodeposited from them, spawned a new field of battery research.
Social Impact: Lithium batteries are widely used in both consumer and defense applications, such as cellular telephones and notebook computers, but such batteries remain expensive. DOE applied research programs are developing new and less costly versions of rechargeable lithium batteries for use in electric and hybrid vehicles.
Reference: Dudney NJ, Bates JB, Lubben D, "Thin-Film Rechargeable Lithium Batteries," in Role of Ceramics in Advanced Electrochemical Systems. American Ceramic Society, 1996 p. 113.
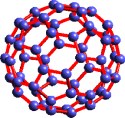
Several lines of research—in spectroscopy, astronomy, and metallic clusters—converged in 1985 to lead to the discovery of an unusual molecule. This cluster of 60 carbon atoms was especially stable because of its hollow, icosahedral structure in which the bonds between the atoms resembled the patterns on a soccer ball. The molecule was named Buckminsterfullerene after the geodesic domes designed by architect Buckminster Fuller. The identification of this form of carbon (also called buckyballs) sparked broad interest in the chemistry of an entire class of hollow carbon structures, referred to collectively as fullerenes. Formed when vaporized carbon condenses in an atmosphere of inert gas, fullerenes include a wide range of shapes and sizes, including nanotubes of interest in electronics and hydrogen storage. The initial discovery was recognized by the 1996 Nobel Prize in Chemistry, awarded to Richard E. Smalley and Robert F. Curl, both supported by the Office of Science, and Curl's colleague Sir Harold W. Kroto of Great Britain. More recently, scientists at Lawrence Berkeley National Laboratory reported a new synthetic method for producing, extracting, and purifying a cluster of 36 carbon atoms in quantities useful for research purposes; they also confirmed the high reactivity and other unusual electrical and chemical properties of this material.
Scientific Impact:The discovery of fullerenes launched a new branch of chemistry, and related studies have contributed to growing interest in nanostructures in general and the principles of self-assembly. Fullerenes also have influenced the conception of diverse scientific problems such as the galactic carbon cycle and classical aromaticity, a keystone of theoretical chemistry.
Social Impact:Fullerenes are highly versatile (there are literally thousands of variations) and thus have many potential applications. For example, fullerene structures can be manipulated to produce superconducting salts, new three-dimensional polymers, new catalysts, and biologically active compounds.
Reference:"C60: Buckminsterfullerene," H.W. Kroto, J.R. Heath, S.C. O'Brien, R.F. Curl, and R.E. Smalley, Nature 318, 162, November 14, 1985"
Plants and microbes are natural biochemical factories, producing important chemicals and materials. (Petroleum deposits are the altered remains of prehistoric plants and microbes.) The Office of Science long has supported basic studies on biochemistry and genetics that are providing insights into how plants and microbes can be modified to make more products with economic value. Christopher Somerville, while at DOE's Plant Research Laboratory at Michigan State University, demonstrated the capability to transfer an alignment of genes from bacteria to higher plants that confer the ability to synthesize biodegradable plastic components. He also studied the biosynthetic pathways for plant oils to learn what genetic changes would produce a different and more desirable type of oil. Research by Lonnie Ingram at the University of Florida focused on the regulation of genes that play critical roles in a bacterium's natural production of ethanol. He engineered DNA with genes for making two key enzymes; not only did this DNA alter the production pathway, but it also was incorporated into the genetic material of numerous other bacteria that did not normally form ethanol—and they started to make it.
Scientific Impact: Somerville's work represents an early breakthrough in enhancing the use of plants as biosynthesizers of precursors for biodegradable plastics, which could replace products now derived from petroleum. Ingram's research suggests the potential for altering many bacteria, with many potential growth substrates, to produce ethanol.
Social Impact: Biosynthesis of compounds that can replace petroleum-derived products could reduce U.S. reliance on foreign oil. The University of Florida patented an ethanol-producing organism capable of growing on certain sugars, and an ethanol plant in Louisiana is demonstrating the commercial potential of a process based on this research.
Reference: Buchanan, B.B., W.Gruissem, and R.L. Jones, Biochemistry & Molecular Biology of Plants, American Society of Plant Physiologists ([email protected]), 2001.

For more than 50 years, the Office of Science and predecessor agencies have supported the discovery and study of the actinide elements, in particular the transuranium elements-atoms that are heavier than uranium. Glenn Seaborg and Ed McMillan of the Lawrence Berkeley National Laboratory, 1951 Nobel Laureates in Chemistry for the discovery of plutonium and other actinide elements, began this quest. Today, the Heavy Element Chemistry program continues the pursuit for a fundamental understanding of actinide and fission product chemistry. The discovery and the exploration of the properties of the transactinides, elements heavier than the actinides, is also being undertaken and presents significant challenges since these elements decay to the lighter elements in minutes, seconds, or milliseconds. One of the leading researchers in this area is Darleane Hoffman of Lawrence Berkeley, whose work earned her the National Medal of Science in 1997 (the nation's highest scientific honor) and the Priestly Medal of the American Chemical Society in 2000. Hoffman contributed to the development of "atom at a time" chemistry which makes possible the study of heavy elements with half-lives of a minute or less. She was among the researchers to confirm the existence of the element seaborgium, named after Seaborg. Hoffman now is involved in an international collaboration to study the chemistry of the transactinides, work inspired by predictions of unexpected chemical properties caused by relativistic effects.
Scientific Impact: Research on the heavy elements yields the basic knowledge that can be used to develop new technologies and processes for the safe handling and disposition of these radioactive materials. For the transactinides, new "atom at a time" chemical techniques are being used to determine and compare their chemical properties to other known elements. The Office of Science heavy element chemistry program is the nation's sole effort addressing the fundamental science of the transuranium elements.
Social Impact: This research helps DOE carry out what is perhaps its most important and difficult responsibility—stewardship of the nation's nuclear science and technology. Studies of these elements and their fission products are needed to address the environmental consequences of the weapons programs and possible accidental release of nuclear materials.
Reference: http://www.nobel.se/chemistry/laureates/1951/seaborg-bio.html
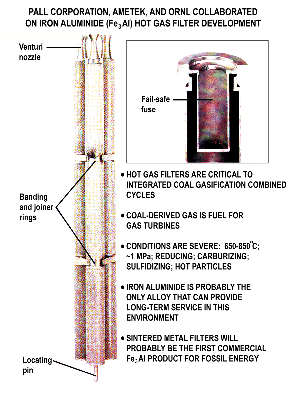
Intermetallic compounds (metallic materials composed of definite proportions of two or more elemental metals) resist oxidation and remain strong at high temperatures, making them useful for energy technologies. But until recently, these compounds were too brittle to be fabricated into conventional shapes using traditional methods. In 1981, Oak Ridge National Laboratory started a program to increase understanding of intermetallic compounds and develop scientific principles for improving their properties. Following a Japanese report suggesting that small amounts of boron made a nickel aluminide compound more ductile, Oak Ridge researchers led by Chain T. Liu determined the mechanism behind the change. They also showed that iron aluminides are intrinsically ductile at ambient temperatures and that brittleness is caused by moisture in the air. Quantum mechanical calculations demonstrated a mechanism that reduced the cohesive strength of atomic layers in these alloys by 70 percent, a discovery that led to new and improved alloy designs. Liu was awarded the 2001 Acta Metallurgica Gold Metal for his outstanding leadership and achievements in this research. The Office of Science then worked with DOE offices of Energy Efficiency and Fossil Energy to fund a research program on intermetallic compounds, an effort that has won three R&D 100 awards from R&D Magazine recognizing significant new technologies, and has resulted in more than 16 patents and 12 licenses.
Scientific Impact: Materials and processing research at Oak Ridge has increased scientific understanding of intermetallic compounds. This work overcame the brittleness problem and improved manufacturability, thus making it practical to use nickel and iron aluminides for high temperature engineering applications.
Social Impact: This research has helped to improve product quality and reduce costs. For instance, the use of nickel-aluminide dies for the hot forging process improves the quality of steel parts in automobiles, and iron-aluminide filters used to remove ash particles during coal gasification reduce costs and resist the corrosiveness of hydrogen sulfide in the gas stream.
Reference: Pope, D. P., C, T. Liu, S. H. Whang, and M. Yamaguchi, eds., High Temperature Intermetallics, Elsevier, New York (1997).

Since the 1940s, Oak Ridge National Laboratory has played a leading role in the development of ion beam technology and its application in materials processing and characterization. A key advance was made in the early 1960s when, in one of the first applications of computers in materials science, researchers predicted that positive ions (charged atoms) moving through a crystal would follow channels between the rows of atoms, thereby penetrating well into the crystal structure. The "ion channeling" effect became the basis for valuable scientific and commercial processes used to force ions into materials as a means of tailoring or altering their properties. One such process is ion implantation, now developed into a fine art that relies on accelerators to drive selected ions into materials at precise distances. Many materials so modified are now in routine use. Today, Oak Ridge operates a facility where the broader scientific community carries out fundamental research on various ion beam techniques to selectively design the near-surface properties of materials.
Scientific Impact: Ion beam techniques are widely used for research on topics such as superconductivity, thin-film electrolytes, quasicrystals, and surface structure and chemistry. The science continues to evolve; new approaches to controlling the morphology and properties of ion-implanted materials and layers now are being developed based on defect physics.
Social Impact: Ion implantation is used extensively in the electronics industry to "dope" semiconductors with special properties, both chemically and spatially. The process is also used to improve the wear resistance of titanium alloys in artificial prostheses for hip and knee replacements. By eliminating the need to rework failed replacement joints, this technology spares individuals from additional surgeries and saves as much as $100 million per year.
Reference: E. Chason, et al, "Ion beams in silicon processing and characterization," Journal of Applied Physics, vol. 81, no. 10, pp. 6513-6561 (1997) [Report of BES study panel]
A. Agarwal, H.-J. Gossmann, D. J. Eaglesham, S. B. Herner, A. T. Fiory, and T. E. Haynes, "Boron-enhanced diffusion of boron from ultra-low energy ion implantation," Applied Physics Letters vol. 74, pp. 2435-2437 (1999).
J. M. Williams and R. A. Buchanan, "Ion implantation of surgical Ti-6Al-4V alloy," Materials Science and Engineering vol. 69, pp. 237-246 (1985).
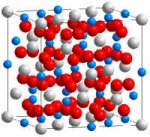
Worldwide, nuclear energy and weapons programs have created 1,350 metric tons of plutonium, an amount still growing by 70 metric tons annually. A major issue facing society is how to dispose safely of plutonium, which is radiotoxic and decays very slowly (it has a half-life of 24,500 years). One strategy is to immobilize it in chemically durable materials that absorb harmful neutrons and resist radiation damage. A 20-year collaboration between Rod Ewing at the University of Michigan and Bill Weber of Pacific Northwest National Laboratory has identified such materials. Using simulation techniques, they discovered that gadolinium zirconate materials resist radiation damage for millennia. These compounds absorb energy through the rearrangement of atoms within the crystal structure without becoming amorphous or structurally unstable—making them superior to the titanate materials being considered internationally for plutonium immobilization. (Plutonium-bearing titanates would degrade much faster.) The researchers also confirmed the mobility of the disturbed atoms and the ease of incorporating plutonium into the gadolinium-zirconate structure.
Scientific Impact: These studies demonstrated a systematic increase in radiation resistance as zirconium is substituted for titanium in gadolinium compounds. Discovery of these materials has stimulated research elsewhere, including Los Alamos National Laboratory, and led to identification of a phase that seems to be the best candidate for immobilizing plutonium.
Social Impact: This material offers a promising means of keeping future generations safe from the dual threats of radioactive contamination caused by plutonium decay, and the nuclear proliferation that might result from further use of the plutonium in weapons. Thus, this work may help resolve major dilemmas of the nuclear age.
Reference: S. X. Wang, B. D. Begg, L. M. Wang, R. C. Ewing, W. J. Weber, and K. V. Govidan Kutty, "Radiation Stability of Gadolinium Zirconate: A Waste Form for Plutonium Disposition," J. Materials Research 14 [12] (1999) 4470-4473.
W. J. Weber et al., "Radiation Effects in Crystalline Ceramics for the Immobilization of High-Level Nuclear Waste and Plutonium," J. Materials Research, 13 [6] (1998) 1434-1484.
W. J. Weber and R. C. Ewing, "Plutonium Immobilization and Radiation Effects," Science 289 (2000) 2051-2052.
B. D. Begg, N. J. Hess, D. E. McCready, S. Thevuthasan, and W. J. Weber, "Heavy-Ion Irradiation Effects in Gd2(ZrxTi1-x)2O7 Pyrochlores," J. Nuclear Materials 289 [1-2] (2001) 188-193.
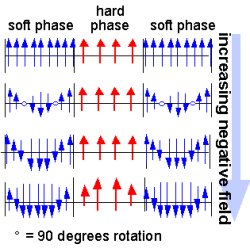
Metallic magnetism is a time-honored field of study that in recent years has undergone a renaissance, thanks in part to Office of Science support. Research at Argonne National Laboratory on thin-film metallic multilayers is widely recognized as providing the basis for the discovery by others of giant magnetoresistance (GMR), an effect used widely today in recording heads in magnetic data storage devices. Related research has resulted in new GMR materials and structures, as well as contributions to the development and understanding of colossal magnetoresistance, a more powerful effect that may be used in future recording devices. Research at Brookhaven National Laboratory and Idaho National Engineering and Environmental Laboratory on hard magnets (permanently magnetized materials) explained the link between microstructure and properties in magnets made of rare earth materials; magnetic properties were improved dramatically through the design of microstructures on the nanoscale. Other work focuses on understanding and exploiting mixtures of hard and soft magnets with high magnetic strength. (The magnetization in soft magnets can be changed with applied magnetic fields.)
Scientific Impact: These studies have advanced the science of magnetic materials and paved the way for manufacture of magnet structures with greater mechanical strength and stability. Researchers benefit from these materials through their use in permanent magnet devices at Office of Science-supported synchrotrons and most other light sources around the world.
Social Impact: Magnetic materials are used in many industrial and consumer devices such as motors, generators, and computers. Improvements in magnet properties and processing characteristics will enhance energy efficiency; for example, the use of rare earth magnets in more efficient electric motors could save the nation several billion dollars annually.
Reference: L. H. Lewis, A. R. Moodenbaugh, D. O. Welch and V. Panchanathan, "Stress, Strain and Technical Magnetic Properties in "Exchange-Spring" Nd2Fe14B + a-Fe Nanocomposite Magnets", J. Phys. D.: Appl. Phys. 34 (2001) 744-751.
D. J. Branagan, M. J. Kramer, Yali Tang, R. W. McCallum, D. C. Crew and L. H. Lewis, "Engineering Magnetic Nanocomposite Microstructures", J. Materials Science, 35(14): 3459-3466, July 2000.
E. E. Fullerton, C. H. Sowers, J. E. Pearson, X. Z. Wu, D. Lederman, and S. D. Bader, "A General Approach to the Epitaxial Growth of Rare-Earth-Transition-Metal Films," Appl. Phys. Lett. 69, 2438 (1996).
E. E. Fullerton, M. J. Conover, J. E. Mattson, C. H. Sowers, and S. D. Bader, "Oscillatory interlayer coupling and giant magnetoresistance in epitaxial Fe/Cr(211) and (100) superlattices", Phys. Rev. B 48, 15755 (1993).
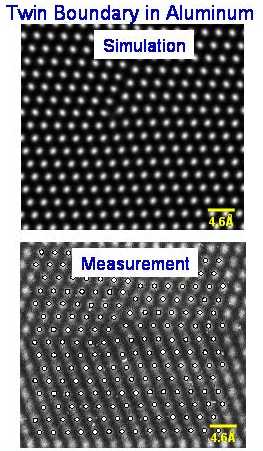
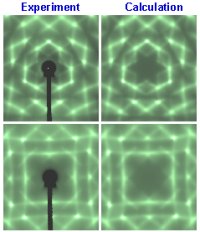
The Embedded Atom Method (EAM) can reliably simulate not only atomic structure of perfect crystals, but also defects and grain boundaries. An example is shown above where the high resolution TEM measurement of an Aluminum grain boundary is directly compared to the EAM calculation.
For many years, scientists did not fully understand how and why metals and alloys sometimes cracked. The mystery was finally solved in the early 1980s, when Murray Daw and Mike Baskes at Sandia National Laboratories developed a way to accurately describe the embrittlement of steel by hydrogen or other corrosive impurities, a problem of interest in defense applications. Using concepts from density functional theory, they constructed the embedded atom method (EAM) from a "first principles" quantum mechanical framework for describing metal bonding. The EAM simplified the quantum behavior so that high-speed calculations could easily be made on very large systems containing hundreds of thousands of atoms. Whereas most fracture studies previously had been conducted on scales of inches or even feet, the EAM revealed much finer detail by focusing instead on atomic-scale processes, such as slight movements of electrons that weaken metal bonds. The EAM accurately describes important quantities such as cohesion and deformation of metals, making possible computer simulations that are useful in designing and predicting the behavior of complex materials and engineering components.
Scientific Impact: The EAM revolutionized computational materials science by enabling large-scale simulations of the atomic structure and evolution of metals; the method successfully simulates complex processes such as metal deformation, embrittlement, and fracture. The method is currently used by more than 100 groups worldwide and has resulted in more than 1,000 published works with more than 2,700 citations to the original paper.
Social Impact: The EAM is used in industry to design alloys for use in metallic parts and products.
Reference: "Parallel Molecular Dynamics With the Embedded Atom Method", S. J. Plimpton and B. A. Hendrickson, in Materials Theory and Modelling, edited by J. Broughton, P. Bristowe, and J. Newsam, MRS Proceedings 291, Pittsburgh, PA, 1993, p 37.

Once considered to be a scientific impossibility, organic magnets (containing little or no metallic material) were discovered by chemist Joel Miller then at Du Pont, and physicist Arthur Epstein of The Ohio State University and both supported by the Office of Science. In 1986, they discovered the first organic material to become magnetically ordered (at very low temperature, -268 degrees C / -441 degrees F), demonstrating for the first time that a magnet could be made using organic chemistry and without the usual high temperature, energy intensive processing. (Magnetic ordering refers to the orientation of each atoms' electron spins, which behave like tiny magnets; when many adjacent electron spins align in the same direction, the material can be a strong magnet.) Miller and Epstein's compound is composed of molecular units. These are the first soluble as well as nonmetallurgically prepared magnets, and are more magnetic then iron metal. (Due to the high density of iron, organic magnets can never be as magnetic as iron on a volume basis.) Their research led to the 1991 discovery of the first organic/polymeric material to exhibit magnetism above room temperature, opening the door to many potential applications. More recently, the two researchers made thin magnetic films using a unique low-temperature process as well as another material that becomes magnetically ordered far above room temperature (~100 degrees C, or 212 degrees F). These accomplishments have been profiled on the covers of 15 journals and recognized by the American Chemical Society's 2000 National Award for Chemistry of Materials.
Scientific Impact: This work created a new class of materials and a thriving field of research that could lead to many new technologies. Since the original discovery, several new classes of polymeric organic magnets have been identified, and research consortiums have formed in both Europe and Japan.
Social Impact: Organic magnets are lighter, more flexible, and less energy intensive to make than conventional metal and ceramic magnets. Applications now being pursued include magnetic shielding, magneto-optical switching, and "smart" materials. The magnetic properties of these materials change when exposed to light, making them candidates for high-density optical data storage systems.
Reference: Ferromagnetic Properties of One-Dimensional Decamethylferrocenium Tetracyanoethylenide (1:1): [Fe(C5Me5)2] + [TCNE]. J.S. Miller, J.C. Calabrese, A J. Epstein, R.W. Bigelow, J. H. Zhang, W.M. Reiff, J. Chem. Soc. Chem. Commun. 1026-1028 (1986).
Organic and Organometallic Magnetic Materials - Designer Magnets, J. S. Miller, A. J. Epstein, Angew. Chem. internat. edit. 33, 385-415 (1994).
Designer Magnets, J.S. Miller, A. J. Epstein, Chem. Eng. News, 73(#40), 30-41 (1995).
A Room Temperature Molecular/Organic-Based Magnet, J.M. Manriquez, G.T. Yee, R.S. McLean, A. J. Epstein, J. S. Miller, Science, 252, 1415-1417 (1991).
Tetracyanoethylene-based Organic Magnets, J.S. Miller, A.J. Epstein, J. Chem. Soc., Chem. Commun. 1319-1325 (1998).
Thin Film V[TCNE]x Magnets, K. I. Pokhodnya, A. J. Epstein, J. S. Miller. Adv. Mater. 12, 410-413 (2000).
Enhancement of the Magnetic Ordering Temperature (and Air Stability) of a Mixed Valent Vanadium Hexacyanochromate(III) Magnet to 99 C (372 K), O. Hatlevik, W. E. Buschmann, J. Zhang, J. L. Manson, J. S. Miller, Adv. Mater. 11, 914-918 (1999).
Organometallic- and Organic-based Magnets: New Chemistry and New Materials for the New Millennium, J. S. Miller, Inorg. Chem. 39, 4392-4408 (2000).
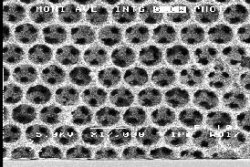
Not long ago, theory and experiments failed to agree on the question of how light propagates in crystals. But in 1990, researchers at Ames Laboratory proved the theorists correct by demonstrating the existence of structures with a "photonic bandgap" (PBG), a range of frequencies within which a specific wavelength of light is blocked. Scientists then knew they could custom-design crystals to trap and manipulate light, sending it down assigned routes and even around loops and bends. Among their novel optical properties, PBG crystals can manipulate light without absorption; the energy not emitted in one frequency region is redirected into other frequencies, a useful feature in energy-efficient devices. Early photonic crystals had a bandgap in the microwave region of the electromagnetic spectrum. Using a layered lattice design and microfabrication capabilities at Sandia National Laboratories, scientists moved the bandgap to shorter wavelengths, in the infrared, for applications such as optical communications. Ames also produced computer programs that allow for the rapid design, analysis, and optimization of PBG structures.
Scientific Impact: The Ames' work spawned a growing global research community and knowledge base focusing on PBG crystals and related atomic properties and behavior. The high accuracy of Ames? theoretical calculations assists in the interpretation and design of PBG experiments and devices, and the layered lattice approach has been used to make the smallest PBG crystal ever fabricated.
Social Impact: PBG crystals could revolutionize the control of light propagation, emission, and absorption in optical devices; thus, they have many potential uses in compact and efficient sensors, antennas, lasers, electronics, lighting, solar cells, and telecommunications equipment (e.g., optical switches, waveguides). The microfabrication method developed at Sandia is economical and lends itself to mass production.
Reference: "Photonic Crystals," M.M. Sigalas, R. Biswas, G. Tuttle, C.M. Soukoulis, and K.M. Ho, Wiley Encyclopedia of Electrical and Electronic Engineering, Volume 16, 345 (John Wiley, 1999).
"Existence of a photonic gap in periodic dielectric structures," K. M. Ho, C. T. Chan, and C. M. Soukoulis, Phys. Rev. Lett. 65, 3152 (1990).
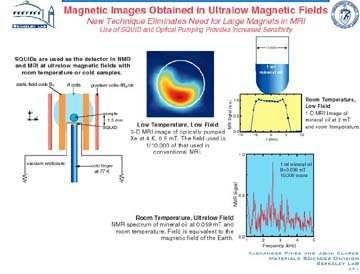
Scientists at Lawrence Berkeley National Laboratory, led by Alex Pines, are world leaders in magnetic identification and imaging concepts and techniques used worldwide in science and industry. Both nuclear magnetic resonance (NMR) spectroscopy and magnetic resonance imaging (MRI) are based on the tiny magnetic moments produced by the "spin" of atomic nuclei; NMR provides spectra for use in identifying molecules, whereas MRI produces recognizable images. The Pines group's research has extended the applicability of NMR to a wide range of problems and materials, including biological systems. They also applied their new methods to study novel materials such as nanocrystals, liquid crystals, and zeolites. Recently, they helped develop a technique in which hyperpolarized gas molecules transfer added momentum to other atomic nuclei, increasing the sensitivity of NMR of molecules in solution and MRIs of materials and organisms, a major step toward extending the power of these techniques in chemistry and biology. The group also developed methods that allow for the use of very low magnetic fields in MRI and may eliminate the need for large, costly magnets in these instruments.
Scientific Impact: These novel concepts and techniques have revolutionized the study of structure, dynamics, and function in solid materials and other systems that previously were inaccessible to NMR investigations. Low-field MRI is ideal for studying highly porous, magnetic materials and fossils and rocks.
Social Impact: These techniques and instruments have been licensed and incorporated into commercial NMR technology used worldwide. A company has licensed the low-field MRI technology to develop medical applications. In addition, Pines has trained about 200 scientists, of whom many now hold leading positions in academia and industry.
Reference: Y.Q. Song, B.M. Goodson, and A. Pines, "NMR and MRI Using Laser-Polarized Xenon," Spectroscopy, (14) 26-33 (July 1999).
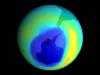
The stratospheric layer of ozone 15 to 50 kilometers above the Earth absorbs ultraviolet radiation, preventing it from reaching the planet's surface. For many years, scientists assumed that this protective ozone would not be affected by release into the atmosphere of chlorofluorocarbons (CFCs), chemically inert and nontoxic gases once common in aerosol sprays and refrigerants. But in fact, CFCs do threaten the ozone layer, as explained in 1974 by F. Sherwood Rowland of the University of California, Irvine, and Mario Molina of the Massachusetts Institute of Technology. Rowland was supported by predecessors to the Office of Science for his research in hot-atom chemistry. Initially interested in species formed as a result of nuclear reactions, he extended his work to study the photochemical formation of chlorine atoms. Roland and Molina theorized that CFC molecules could be split apart by solar radiation to produce chlorine atoms, which could catalyze the destruction of ozone. They were right, as underlined later by discovery of the "ozone hole" over the Antarctic. Rowland and Molina, together with Paul Crutzen of the Max-Planck-Institute for Chemistry in Germany, shared the 1995 Nobel Prize in Chemistry for their work on the formation and decomposition of ozone.
Scientific Impact: Discovery of the effect of CFCs on the ozone layer was a seminal contribution to atmospheric chemistry.
Social Impact: Rowland and Molina's work initially led to restrictions on CFC releases; after discovery of the ozone hole, an international agreement was signed to limit the manufacture and use of these compounds. Thus, this research has helped mitigate a global environmental problem with potentially catastrophic consequences. It will take at least 100 years for the ozone layer to recover fully.
Reference: Molina, M. J., and F. S. Rowland, Stratospheric Sink for Chlorofluoromethanes: Chlorine Catalyzed Destruction of Ozone, Nature, 249, 810-814 (1974)

TEM Structure Analysis
The cost of solar electricity has been reduced 100-fold over the past two decades, but further reductions are needed before solar power is widely used. Scientists at the National Renewable Energy Laboratory took major steps toward this goal by designing photovoltaic cells (which convert sunlight to electricity) with 30 percent efficiency, much higher than the 10-20 percent levels achieved previously. The new cells consist of thin layers of semiconductors applied to a low-cost backing, such as glass or plastic. The researchers received Office of Science support to develop a basic understanding of the opto-electronic properties of various semiconductors. Calculations of electronic structure provided the knowledge needed to precisely engineer layered semiconductors. Then, a tandem device was designed with two solar cells made of materials that respond to different parts of the solar spectrum; the top cell (made of gallium indium phosphide) absorbs the high-energy component of sunlight and passes the rest to the bottom cell (made of gallium arsenide) for absorption. Researchers are working on the addition of a third cell to push efficiency to more than 40 percent, to open up new opportunities for terrestrial and space applications.
Scientific Impact: These advances have added to the scientific and engineering knowledge base needed to make solar power more practical and useful. For instance, the material used in the top layer of the new device is much more resistant to radiation damage than conventional silicon and thus will have a longer useful life.
Social Impact: The technology was transferred to a major supplier of photovoltaic cells for space power, and four satellites using it are in orbit, flashing back telephone and television signals. The new solar cells provide as much as 50 percent more power than previous cells, so the satellites can carry more communications links, experiments, or other projects and operate more economically.
Reference: Cotal, H. L.; Lillington, D. R.; Ermer, J. H.; King, R. R.; Karam, N. H.; Kurtz, S. R.; Friedman, D. J.; Olson, J. M.; Ward, S.; Duda, A.; Emery, K. A.; Moriarty, T. (2000). "Highly Efficient 32.3% Monolithic GaInP/GaAs/Ge Triple Junction Concentrator Solar Cells." Program and Proceedings: NCPV Program Review Meeting 2000, 16-19 April 2000, Denver Colorado. BK-520-28064. Golden, CO: National Renewable Energy Laboratory; pp. 111-112; NICH Report No. CP-520-29664.
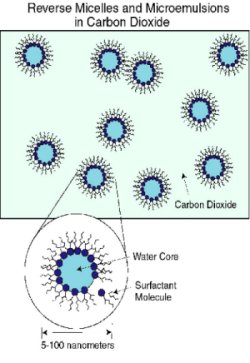
For many decades, the Office of Science and predecessor agencies have supported studies of the scientific principles underlying chemical separations and analysis. The most notable achievement was the development of the host-guest complexation concept by Donald J. Cram of the University of California, Los Angeles. This concept, which explains how molecules recognize and react with each other, changed how scientists think about separation and sequestration of elements. Cram also synthesized organic molecules that imitated the primary functions of enzymes, helping to fulfill a long-standing dream of chemists. For his pioneering work, Cram shared the 1987 Nobel Prize in Chemistry with two other scientists. Other researchers with Office of Science support developed inductively coupled plasma to produce sample materials for chemical analysis; wrote a powerful software program, SIMION, to help design particle beams and traps for fundamental studies; performed important basic research on supercritical fluids (liquefied gases used in solvent extraction); and developed laser-based detection schemes that enhanced the sensitivity of important analytical methods.
Scientific Impact: Cram helped to lay the foundation for one of the most active fields of chemical research, known as host-guest or supramolecular chemistry, which is directly applicable to separations. The work on inductively coupled plasma ushered in the era of ultra-trace multi-element analysis, enabling the rapid and accurate determination of up to 70 elements in metals, alloys, and organic compounds (such as oil, serum, blood, and soils). SIMION is used at every national laboratory.
Social Impact: Inductively coupled plasma is used in environmental testing and the production of ultrapure materials for the semiconductor and nuclear industries. The research on supercritical fluids contributed to the recent introduction of "green chemistry" for commercial dry cleaning and polymer manufacturing. SIMION is used by instrument suppliers that design mass spectrometers.
Reference: DOE-BES Chemical Sciences, Highlights of Progress in Separations Sciences, 1980-1999, Edited by Charles H. Byers, IsoPro International Inc., 2140 Santa Cruz Ave, #C304, Menlo Park, CA 94025

Arabidopsis thaliana
Arabidopsis thaliana, a small plant in the mustard family, has become the model for molecular genetic research on plants because of its small size, rapid growth cycle (6 weeks), large production of seed, and small genome (the smallest known of any flowering plant). In 1990, when Arabidopsis was being tested as a laboratory model for plant genetics, the DOE Plant Research Laboratory at Michigan State University initiated a project to analyze expressed sequence tags (ESTs), mirror images of fragments of genes and the proteins they make. Because they can be used to scan for and tag active genes, ESTs rapidly became important tools for identifying and isolating plant genes. Subsequently, other federal agencies provided support, resulting in a multinational computer database linked to U.S. and European stock centers, which distribute seeds and DNA of Arabidopsis to researchers worldwide. The research that grew out of this work laid the groundwork for the Arabidopsis Genome Initiative, which began 1996 as a multinational effort to sequence this plant's genome. The entire sequence (130 million pairs of chemical units) was officially completed recently and is largely available on the World Wide Web. As a direct model for 250,000 closely related species, Arabidopsis will help scientists understand the molecular basis of plant growth and development and address fundamental questions in plant physiology, biochemistry, cell biology, and pathology.
Scientific Impact: The MSU effort was instrumental in establishing Arabidopsis as a model organism for identifying and studying plant genes at the molecular level. Since the early sequencing of ESTs, studies using Arabidopsis have yielded many significant advances, including the discovery of plant hormone and signal receptor action and components of disease resistance.
Social Impact: Concentrated research on this single plant will provide detailed information that can be applied to a wide range of plant attributes relevant to energy, manufacturing, the environment, agriculture, and even human health. One result has been crops that are more resistant to the cold; further insights will help scientists make other plants easier to grow under adverse conditions, healthier to eat, and more disease resistant.
Reference: Arabidopsis thaliana Genome Sequencing Completed," Nature, December 14, 2000.
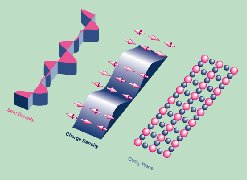
Stacks of ultrathin layers—each less than one-thousandth the thickness of a human hair—are the secret to a class of artificially grown materials that have enabled numerous advances in technology over the past generation. In 1981, scientists at Sandia National Laboratories were the first to predict the unique electronic and optical properties of strained-layer semiconductor (SLS) superlattices, and, a few years later, the first to make devices from them. These crystalline materials got their name because the spacing between the atoms in different layers is mismatched initially, but the thinness of the layers allows alignment by elastic strain without causing dislocations or other defects. Because the number, composition, and thickness of the layers can be varied over wide limits, scientists can tailor the electrical and optical properties to design materials and devices with targeted properties. This work has won a number of awards, including the American Physical Society's International Prize for New Materials in 1993.
Scientific Impact: This work established new areas of materials science and electronics as well as new research technologies; for instance, SLS materials are used to make transistors for high-frequency, low-noise electronic amplifiers, such as those found in radiotelescopes. These materials made it possible for scientists to tailor the wavelength (or color) of light-emitting devices (such as light-emitting diodes) and increase the speed of electrons in transistors.
Social Impact: The SLS technology revolutionized the multibillion-dollar field of opto-electronics and is a key to wireless communications. These materials enhance the performance and efficiency of semiconductor lasers and make possible new types of lasers with applications in optical communications, supermarket scanners, remote sensing, and medical diagnostics.
Reference: "Laser Gain and Threshold Properties in Compressive-Strained and Lattice-Matched GaInNAs/GaAs Quantum Wells", W. W. Chow, E. D. Jones, Appl. Phys. Lett 75, pp. 2891-93 (1999).
"Pressure Dependence of the Bandgap Energy and the Conduction-Band Mass for an N-Type InGaAs/GaAs Single-Strained Quantum Well", E. D. Jones, S. W. Tozer, and T. Schmiedel, Physica E 2, pp.146-150 (1998).
"Study of Cyclotron Resonance and Magneto-Photoluminescence of N-Type Modulation-Doped In GaAs Quantum Well Layers and Their Characterizations", N. Kotera, E. D. Jones, K. Tanaka, H. Arimoto, M. Ohno, N. Miura, T. Mishima, edited by S. C. Shen, D. Y. Tang, G. Z. Zheng, and G. Bauer (World Scientific, Singapore, 199) pp. 591-598.
"Room-Temperature Continuous Wave InGaAsN Quantum Well Vertical-Cavity Lasers Emitting at 1.3 Microns", K. D. Choquette, J. F. Klem, A. J. Fischer, O. Blum, A. A. Allerman, I. J. Fritz, S. R. Kurtz, W.G. Breiland, R. Sieg, K. M. Electronics Letters Vol. 36, 1388 (2000).
"GaAsSb/InGaAs Type-II Quantum Wells for Long-Wavelength Lasers on GaAs Substrates", J. F. Klem, O. Blum, S. R. Kurtz, I. J. Fritz, and K. D. Choquette, J. Vac. Sci. Technol. B, Vol. 18, 1605 (2000). "Strained-layer semconductor superlattices from lattice mismatched materials." Osbourn, J.C. J. Applied Physics (53) p1586 (1982).
"InGaAs strained-layer semiconductor superlattices: A proposal for useful new electronic materials." Osbourn, J.C. Phy Rev. B. (27) p5126 (1983).
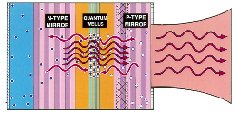
Since the discovery in the 1980s of high-temperature superconductors, the Office of Science has supported research designed to explain and improve the physical behavior of these materials and develop methods of making wires and other objects from them. These materials conduct electricity with virtually no resistance at temperatures high enough to be cooled by liquid nitrogen (-196 degrees C, or -321 degrees F) instead of more costly helium. Studies at various national laboratories have led to discoveries concerning, for example, the relationships between magnetic behavior and superconductivity, and between material layering and current-carrying capability. Argonne National Laboratory clarified the nature of several different phases of vortex matter (compounds often break down at the vortex, where the molecules of different materials meet), leading to new configurations that improve conductivity. Argonne also built the first superconducting motor and developed a process for welding lengths of wire in a way that maintains superconductivity. Other investigators have observed "charge stripes" in materials exhibiting colossal magnetoresistance, an unusual and powerful effect that may be exploited in future magnetic recording devices. Years of research at Oak Ridge National Laboratory led to the development of processes that may enable the manufacture of long lengths of superconducting wires and tape.
Scientific Impact: This research has greatly increased scientific understanding of high-temperature superconductors. As yet, there is no comprehensive theory that explains all of the experimental phenomena; this remains a key question in condensed matter physics.
Social Impact: Superconducting wires and tape can carry 100 to 200 times more electric current than conventional wires. These innovations could enable the widespread commercialization of more efficient types of power generation, transmission, and electrical equipment and devices, offering tremendous energy savings and emissions reductions.
Reference: S.L. Bud'ko, G. Lapertot, C. Petrovic, C.E. Cunningham, N. anderson, and P.C. Canfield. "Boron Isotope Effect in Superconducting MgB2," Physical Review Letters, February 26, 2001.
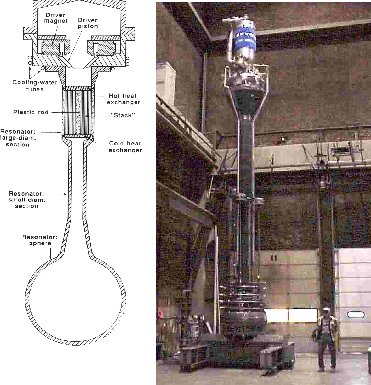
Thermoacoustic Refrigeration
A sound wave consists of oscillations in pressure, temperature, and displacement. Although the temperature oscillations are small, research during the past two decades has shown that this "thermoacoustic" effect can be harnessed to produce powerful, reasonably efficient heat engines, including heat pumps, and refrigerators. Thermoacoustic engines typically have no moving parts; at most, there is a single oscillating part (such as a loudspeaker) with no sliding seals. Thus, these engines have the potential to be both simple and reliable. Research by Greg Swift at Los Alamos National Laboratory on the thermodynamics of the thermoacoustic process has led to the development of prototype refrigerators with cooling powers up to tens of watts, and prototype engines with efficiencies approaching those of conventional engines. The research has spawned collaborative efforts that have resulted in advances in the theory, design, and construction of thermoacoustic devices.
Scientific Impact: Los Alamos' leadership in both the scientific and technological aspects of thermoacoustics since the mid-1980s has generated a sizeable academic research community around the world. The first international workshop on thermoacoustics will be held in 2001.
Social Impact: Thermoacoustic energy conversion (including conversion of heat to acoustic power, acoustic power to refrigeration, and acoustic power to mixture separation) is reasonably efficient and should be inexpensive and reliable in mass production. Efforts are under way to develop a natural-gas liquefier for use in remote locations, a residential co-generation system to produce both electricity and gas heat, an electric generator for deep-space probes, and a water chiller for use on submarines.
Reference: S. Backhaus and G.W. Swift. "A thermoacoustic-Stirling heat engine." Nature, 399:335-338, 1999.
G. W. Swift. "Thermoacoustic engines and refrigerators." Physics Today, pages 22-28, July 1995.
G. W. Swift. "Thermoacoustic engines." J. Acoust. Soc. Am., 84:1145-1180, 1988.
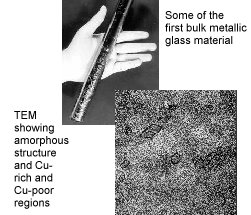
Bulk Metallic Glasses
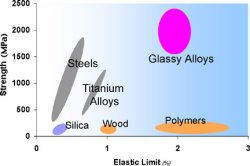
Metallic glasses with extraordinary magnetic properties, and practical methods for processing these materials, have been developed over the past four decades with support from the Office of Science and predecessor agencies. Pol Duwez at the California Institute of Technology produced the first ribbons of metallic glasses, which had unusual mechanical strength, magnetic behavior, and resistance to wear and corrosion that set them apart from conventional crystalline materials. The processing method involved chilling molten metal at rates in excess of 1,000,000 degrees C per second. Duwez and his student William L. Johnson also discovered other alloys that could be made into metallic glasses for high-efficiency magnets, but expensive processing was required to fabricate forms useful for motors and transformers. During the 1980s, Johnson developed new compositions that could be processed without rapid cooling in bulk or three-dimensional form (bulk forms are more than 20 times thicker than the roughly 40-micrometer ribbons), suitable for casting or possibly molding into complex shapes for precision parts, without the costs or wastes associated with machining. Recently, scientists at Los Alamos National Laboratory produced a bulk ferromagnetic glass with a record-low magnetic energy loss that does not require expensive processing, a form appropriate for energy conversion devices.
Scientific Impact: This research opened up a new area of materials science and technology—for which DOE was the sole U.S. supporter until recently—that offers opportunities for increasing the efficiency of magnets, motors, and transformers. Los Alamos is a world leader in research on bulk ferromagnetic glasses, the only form appropriate for motors and transformers.
Social Impact: These materials are used in products ranging from motor components to golf clubs and also have great potential for military applications. The use of bulk ferromagnetic glasses in energy-conversion devices would reduce costly losses from power-distribution systems and corrosion damage, and the consequent reduced use of energy from fossil sources would reduce the rate of release of carbon dioxide into the atmosphere.
Reference: Masuhr A, Busch R, Johnson WL. "Rheometry and Crystallization of Bulk Metallic Glass Forming Alloys at High Temperatures." ISMANAM 1997 - Materials Science Forum. Barcelona, Spain. Switzerland: Trans Tech Publications, 1998: 779-84.
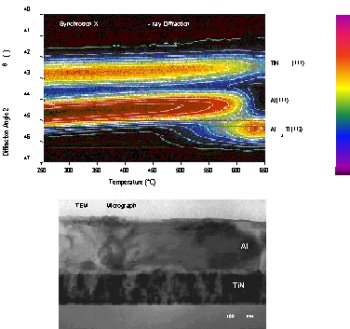
Novel semiconductors, superconductors, and corrosion-resistant materials have been developed recently through nanoscale research on transition metal nitrides. J. E. Greene, I. Petrov, and colleagues at the University of Illinois Seitz Materials Research Laboratory, with Office of Science support, combined theoretical modeling with fundamental growth and characterization experiments to improve the basic mechanical and electrical properties of nitrides. They developed new processes for depositing these materials with control of atomic-scale reaction and diffusion, thereby designing whole families of alloys with unique properties that are impossible to achieve under equilibrium conditions. To achieve these properties, it was necessary to control grain size and texture on a scale on the order of 10 nanometers (nm), and to achieve interfacial widths of 0.1 nm to 1.0 nm. This work has many applications and has been recognized by many awards, including the 1999 David Turnbull Lectureship of the Materials Research Society, the 1998 David Adler Prize from the American Physical Society, and the Tage Erlanger Prize in Physics (the second-ranking Swedish prize in science after the Nobel Prize).
Scientific Impact: This work extended the science of transition metal nitrides, making possible the design of entirely new materials. These achievements also demonstrate the value of research on the nanoscale, an emerging field of great importance.
Social Impact: Transition metal nitrides already have practical uses; titanium aluminum nitride, for example, has become ubiquitous in wear-, corrosion-, and diffusion-resistant coatings for products such as cutting tools. The new alloys have enabled the use of copper interconnects in integrated circuits through the creation of improved diffusion barriers, thus paving the way for a new generation of faster computer chips.
Reference: J. S. Chun, I. Petrov, and J.E. Greene, "Dense fully 111-textured TiN diffusion barriers: Enhanced lifetime through microstructure control during layer growth" J. Appl. Phys., 86 3633 (1999).
D. Gall, I. Petrov, P. Desjardins, and J.E. Greene, "Microstructural evolution and Poisson ratio of epitaxial ScN grown on TiN(001)/MgO(001) by ultrahigh vacuum reactive magnetron sputter deposition" J. Appl. Phys., 86 5524 (1999).
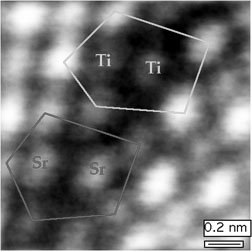
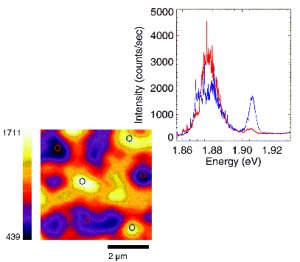
A microscope invented with Office of Science support is the first technique to produce a direct image of a complex atomic structure while also identifying the atoms involved. Steve Pennycook of Oak Ridge National Laboratory combined elements of three existing electron microscopes to make the Z-contrast microscope, which uses electrons bounced off (scattered from) a sample to form an image of the atoms. Because the scattered intensity depends on the atomic number (Z) of the chemical element being probed, the image intensity provides a means of identifying the atoms. The method improves on scanning electron microscopes (which produce clear images but cannot penetrate materials), transmission electron microscopes (which produces images that cannot be interpreted directly as atomic structure), and the hybrid scanning transmission electron microscope (which produces outstanding microanalysis but poor-quality images). Z-contrast microscopy is particularly suited to the viewing of interfaces, grain boundaries, and defects in materials-features that cannot be analyzed well using indirect means. The Z-contrast microscope won an R&D 100 award from R&D Magazine as a significant new technology. Pennycook also received the Materials Research Society Medal and the Kurt J. Heinrich Award of the Microbeam Analysis Society.
Scientific Impact: Z-contrast microscopy has had major impact on the study of materials structure. It has achieved the highest resolution of a crystal structure ever recorded in a microscope and provided new information on the atomic-scale structure and chemistry of a variety of materials—correcting previously published quasicrystal structures, for example.
Social Impact: Z-contrast microscopes are commercially manufactured. This tool is likely to lead to dramatic advances in structural materials, superconductors, and semiconductors, especially in the smoothness of interfaces where different materials join, and thereby pave the way for improved computers, fiber-optic communications, medical imaging, and laser-disc players.
Reference: M. F. Chisholm and S. J. Pennycook, "Z-Contrast Imaging of Grain-Boundary Core Structures in Semiconductors," MRS Bulletin 22, 53 (1997).




