User Facilities: Tools for Seeing Atoms
Revealing the atomic-scale secrets of materials requires a variety of cutting-edge imaging and analytical instruments that use beams of different types of particles or radiation. These beams of x-rays, neutrons, and electrons provide complementary information about various aspects of a material’s nanoscale structure. These techniques together are revealing insights that enable us to understand and ultimately control the properties of materials.
Download High Resolution Image
Building on investments made by the Department of Energy (DOE) and its predecessor agencies in pioneering work to develop nuclear reactors and particle accelerators, the DOE Office of Science today provides the research community with a suite of scientific user facilities—x-ray light sources, neutron sources, and electron-beam microcharacterization centers—to explore the atomic world. Since their inception in the 1970s, the growth of scientific user facilities worldwide has been remarkable. Major scientific user facilities make possible experimental studies that cannot be done in ordinary laboratories, and these facilities have created a new style of research in which scientists conduct investigations that benefit from a merging of ideas and techniques from different disciplines.
Together, these DOE facilities help more than 10,000 researchers pursue discoveries that improve the economy, energy options, medical treatments, technologies, and our fundamental understanding of the materials that make up our world. The leading-edge research conducted at these facilities has earned numerous awards, including Nobel prizes in chemistry, physics, and medicine. Selected facilities are described below.
X-Ray Light Sources
Each 2-m-long, 1-ton undulator at LCLS contains many “teeth,” or alternating pairs of north-south magnets that force electrons to follow a wavy path, creating x-rays.
Millions of times brighter than medical x-rays, light sources generate high-quality, stable beams of x-rays and other electromagnetic radiation that can be used for numerous experimental techniques. Electrons are deflected as they travel past bending magnets along the circular track of a synchrotron or through other specially designed magnetic devices. This creates a continuous spectrum of radiation with various wavelengths and strengths (e.g., ultraviolet light and hard or soft x-rays) that scientists can select to suit their particular experiment. These rays are then sent down pipes called beamlines to sophisticated instruments in work areas or end stations where studies are conducted.
The Advanced Photon Source (APS) is the largest scientific user facility in the United States (its electron storage ring could encircle a major-league baseball stadium). By bending accelerated electrons into a circular path with magnetic fields, the APS produces ultrabright radiation in the hard x-ray range that can be focused onto small samples, allowing researchers to quickly gather large amounts of detailed data. With over 60 beamlines equipped with numerous instruments customized to particular research needs, the APS offers a broad range of experimental capabilities.
The Linac Coherent Light Source (LCLS) is an x-ray free-electron laser that is used much like a super high-speed strobe flash that produces very short pulses of electrons. The LCLS electron beam is linear, and the electrons travel through a 1-kilometer-long accelerator before emitting x-rays as they slalom back and forth within the final 100-meter stretch of undulators. The waves of x-rays become synchronized as they further interact with the electron pulses. The resulting ultrafast pulses of hard x-rays allow scientists to take stop-motion pictures of moving molecules, shedding light on the fundamental processes of chemistry, technology, and life itself.
Neutron Sources
The SNS chamber contains the central liquid mercury target that is bombarded by protons to produce spallation neutrons.
The SNS chamber contains the central liquid mercury target that is bombarded by protons to produce spallation neutrons. Neutrons are produced for scientific experiments in a reactor or using an accelerator- based process called spallation. By bombarding a heavy-element target (e.g., mercury) with accelerated protons, spallation causes some neutrons to be knocked out or “boiled off” as the bombarded nuclei heat up. When the energetic protons collide with the heavy atomic nuclei in the target, about 20 to 30 neutrons are expelled from each nucleus.
Neutrons from these sources are guided through beamlines to the sample material being investigated. The neutrons bounce off the sample, scattering in different directions. Where they bounce, how fast, and where they land reveal details about the sample’s properties. Special detectors measure these scattering patterns and send the data to a computer for various analyses and processing, which often include reconstructing a three-dimensional image of a sample’s nanoscale architecture. Such images provide unique insights into a material’s structure, dynamics, and magnetic properties.
The Spallation Neutron Source (SNS) is a pulsed accelerator-based neutron source. Before hitting a target, protons are accelerated to very high energies and passed into a ring where they accumulate in bunches. Each bunch is released from the ring as a pulse, creating a neutron beam 10 times more intense than any other such source in the world. Each pulse contains neutrons in a range of wavelengths and energies tuned to a specific experiment by moderators. The highest energy neutrons are moderated to room temperature or cooler. Cooling neutrons slows their speed and lengthens their wavelengths to levels suitable for analyzing certain magnetic properties or studying the larger structural components of “soft” materials such as proteins and polymers.
Electron-Beam Microcharacterization
More than a thousand times smaller than neutrons, electrons are tiny, negatively charged particles that can be energized, deflected, or focused into beams by electric and magnetic fields. Electrons interact so strongly with the nuclei and electrons of atoms that they scatter with much higher intensity than x-rays or neutrons, thereby generating powerful signals from small numbers of atoms and even from just a single atom. These attributes make electrons very useful not only for characterizing matter at subnanometer to micrometer scales but also for capturing images of individual atoms within a sample.
The National Center for Electron Microscopy (NCEM) at Lawrence Berkeley National Laboratory is home to the Transmission Electron Aberration-corrected Microscope (TEAM). TEAM is the product of a collaboration among DOE electron-beam experts to develop the first electron microscope to surpass a spatial resolution of 0.05 nanometers, roughly half the diameter of a hydrogen atom. An electron gun atop the TEAM instrument applies an intense electrical field and heat to emit electrons from a source material. The electron gun accelerates and focuses the emitted electrons into a tight beam traveling at more than half the speed of light. The accelerated electrons behave like waves with very short wavelengths. Once shot from the electron gun, the beam is condensed and directed toward the sample. The electron waves zoom through the sample and into a series of magnetic lenses that magnify and focus them to form an image on a screen at the bottom of the microscope column. As they pass through the magnetic lenses, some electron waves are bent at different angles, blurring the image. TEAM components correct these aberrations, giving this instrument its unprecedented capability of directly imaging subatomic distances with ultrahigh precision.
Measurement Techniques for Instruments Using X-Ray, Electron, and Neutron Beams
Imaging
Imaging techniques use particle beams to obtain pictures with fine spatial resolution of materials. Microscopy using electrons or x-rays is now powerful enough to image individual atoms within a structure, reveal local physical properties by measuring small shifts in the positions of atoms, and study molecular events that last only one millionth of a billionth of a second.
Scattering
Scattering or diffraction occurs when a stream of particles or a wave strikes an object and is deflected from its original trajectory. When a particle beam is aimed at a sample, some of these particles will interact with nuclei or with the electrons surrounding nuclei and bounce away at an angle. The scattering pattern created when the “bounced” particles hit a detector gives information about the structure of the sample.
Spectroscopy
Spectroscopy is the study of the interactions between matter and radiated energy. The spectrum of wavelengths or frequencies of energies absorbed or emitted by the sample under investigation contains information about the electronic structure of the matter, such as the nature of its chemical bonds or the behavior of its electrons during excitation.
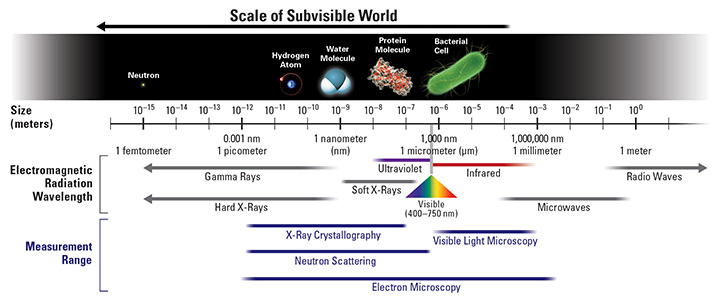
Seeing Matter at Atomic and Molecular Scales
Image credits. Scale of subvisible world and SNS chamber: Oak Ridge National Laboratory. TEAM instrument and micrograph of atoms in alloy: Lawrence Berkeley National Laboratory. Undulator: Linac Coherent Light Source, SLAC National Accelerator Laboratory. X-ray diffraction of virus: T. Ekeberg, Uppsala University. Orbitals in metal oxide: Argonne National Laboratory.










